![]()
- Very good safety profile with no AEs and SAEs related to NBTXR3
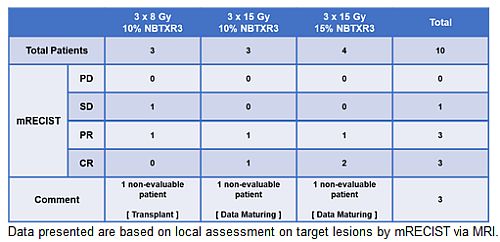
- Complete Response achieved in 3 out of 7 patients evaluable and partial response achieved in 3 out of 7, among 10 patients treated
- Third indication in global NBTXR3 development confirming transferability across different cancers
Paris, France and Cambridge, Massachusetts, USA, Jan 22, 2018 - (ACN Newswire) - NANOBIOTIX (Euronext: NANO - ISIN: FR0011341205), a late clinical-stage nanomedicine company pioneering new approaches to the treatment of cancer, today announces a first readout of intermediate data on the safety and feasibility in its Phase I/II trial evaluating NBTXR3 in liver cancers, including primary (Hepatocellular, HCC) and liver metastasis from other tumors.
Nanobiotix Chief Medical Officer Elsa Borghi, said: "This study successfully demonstrated the safety and the feasibility of the treatment for two different and important indications in liver oncology. These results open a promising pathway for NBTXR3 in patients highly vulnerable."
Population treated
Patients with either HCC or liver metastases frequently cannot undergo surgery and have very few or no therapeutic options available. Radiation therapy has been shown to improve outcomes of these patients. Clinical trials have shown a direct correlation between higher doses of radiation therapy and increased survival, in both patient populations. The delivery of a high radiation dose is complex and cannot be done in an optimal way in most situations due to radiation therapy associated toxicity. NBTXR3 aims to amplify the energy dose within the tumor to offer better clinical results and to improve the poor prognosis of these populations.
Nanobiotix's Phase I/II trial evaluates the safety and preliminary efficacy of NBTXR3 nanoparticles administrated by intra-tumoral (IT) or intra-arterial (IA) injection and activated by high precision radiation therapy, delivered as high dose fractions (Stereotactic Body Radiation Therapy (SBRT)) for the treatment of liver cancers.
Results presented at ASCO GI annual meeting (Abstract #202303)
A phase I/II trial of NBTXR3 nanoparticles activated by SBRT in the treatment of liver cancers. (Poster board ID TPS 551, Authors: Enrique Chajon, Marc Pracht, Thierry De Baere, France Nguyen, Jean-Pierre Bronowicki, Veronique Vendrely, Anne-Sophie Baumann, Valerie Croise-Laurent, Eric Deutsch; Centre Hospitalier Universitaire de Rennes, Rennes, France; Centre Eugene MARQUIS, Rennes, France; Department of Imaging and Therapeutic Imaging, Gustave Roussy and Paris-Sud University, Villejuif, France; Institut Gustave Roussy, Villejuif, France; INSERM 954, CHU de Nancy, Universite de Lorraine, Nancy, France; CHU Bordeaux, Bordeaux, France; Institut de Cancerologie de Lorraine, Nancy, France).
Enrollment was completed for dose levels 10% (6 pts) and 15% (4 pts). The recruitment at 22% which is the third dose level, is ongoing.
1. Primary endpoints: Safety and Feasibility
The product has demonstrated a very good safety profile, with no adverse event (serious or not) related to NBTXR3 occurred and no dose-limiting toxicity (DLT).
Additionally, the NBTXR3 injections have been demonstrated to be feasible with successful injections in all cases, a good dispersion of the product and the confirmation of its permanence within the tumor structure, from the first day until the last day of radiotherapy treatment, with no negative effect related to the treatment.
Importantly, NBTXR3 nanoparticles did not have any impact on the reliability of image-guided radiation therapy (IGRT).
2. Secondary endpoint: Overall response rate to date
Best Overall Response data to date, presented in the table below are based on local assessment on target lesions by mRECIST via MRI.
Out of 10 patients treated, 7 are evaluable. Out of these 7 patients, 3 achieved a complete response and 3 a partial response as best overall response.
These data are only first preliminary response data which will be completed with additional data.
Table: https://www.acnnewswire.com/topimg/Low_Nanobiotix180122.jpg
3. Potential value of NBTXR3 in this indication
The physical mode of action of NBTXR3 may represent a breakthrough approach for the local treatment of liver cancers, as it does not engage liver and renal functions, i.e. nanoparticles are neither metabolized by the liver nor excreted by the kidney, which is key and highly valuable.
4. Next steps
The trial is planned to evaluate patients at 10, 15, 22 and 33% NBTXR3 dose level. The two first levels (10 and 15%) have been completed, and the trial is recruiting patients to be treated at 22%.
NBTXR3 is also being evaluated in: soft tissue sarcoma (STS), head and neck cancers and prostate cancer. Additionally, head and neck cancer and rectal cancer trials led by Nanobiotix's Taiwanese partner, PharmaEngine, are underway in the Asia Pacific region.
About liver cancers
According to WHO, liver cancers are the second most common cause of cancer death in the world with 745,000 deaths each year, and 800,000 new liver cancer patients per year.
Liver cancers are challenging diseases to address. Stereotactic Body Radiation Therapy (SBRT) is the safest and most modern radiotherapy currently available for the treatment of malignant liver tumors but SBRT has been shown to be efficient only in specific subsets of population with small tumors. Complete response is a rare event and local control is often compromised in big tumors, metastases and HCC with portal vein tumor thrombosis and short progression Free Survival and Overall survival.
About NBTXR3 trial protocol in liver cancer
NBTXR3 is a first-in-class radio-enhancer nanoparticle designed for direct injection into malignant tumors. NBTXR3 has the potential to improve radiotherapy efficacy by destroying locally advanced cancers more efficiently. It has been engineered to increase the local absorption of the radiotherapy dose and thereby increasing the efficacy of radiotherapy without increasing toxicity or causing damage to surrounding healthy tissues.
The first phase of the ongoing, multicenter open-label, single-arm study is a dose-escalation to evaluate the safety, feasibility and preliminary clinical activity along with determining the recommended dose of NBTXR3 in this indication. The second phase of the trial will be a dose-expansion phase, which will be a cohort expansion at the recommended dose of NBTXR3.
Patients receive a single injection of NBTXR3 into the tumor or metastasis 24 hours before the beginning of the radiotherapy treatment. The total maximum radiotherapy dose is 45 Gy, delivered as three fractions of 15 Gy each, over 5 to 7 days.
About NBTXR3
NBTXR3 is an injectable aqueous suspension of hafnium oxide nanoparticles designed as an innovative therapeutic agent for the treatment of solid tumors, currently in clinical development by Nanobiotix.
Once injected intratumorally, NBTXR3 can deposit high energy within tumors only when activated by an ionizing radiation source, notably radiotherapy. Upon activation, the high energy radiation is physically designed to kill the tumor cells by triggering DNA damage and cell destruction and improve clinical outcomes.
Promising results indicate that NBTXR3 activity could be applicable across solid tumors triggering immunogenic cell death, leading to an immune response, reinforcing a local and potentially systemic effect, and contributing to transform "cold" tumors into "hot" tumors. NBTXR3's major characteristics are represented by a high degree of biocompatibility, one single administration before and during the whole therapy and the ability to fit into current standards of radiotherapy care.
NBTXR3 entered clinical development in 2011 in a Phase I/II with patients suffering from advanced soft tissue sarcoma of the extremities and is currently in the final stages of its subsequent phase II/III. In parallel, it is currently being tested in numerous Phase I/II clinical trials with patients suffering from locally advanced squamous cell carcinoma of the oral cavity or oropharynx (head and neck), liver cancer (hepatocellular carcinoma and liver metastasis), locally advanced or unresectable rectal cancer in combination with chemotherapy, head and neck cancer in combination with concurrent chemotherapy, and prostate adenocarcinoma.
About NANOBIOTIX: www.nanobiotix.com
Nanobiotix (Euronext: NANO / ISIN: FR0011341205) is a late clinical-stage nanomedicine company pioneering novel approaches to the treatment of cancer. The Company's first-in-class, proprietary technology, NanoXray, enhances radiotherapy energy with a view to providing a new, more efficient treatment for cancer patients.
NanoXray products are compatible with current radiotherapy treatments and are meant to treat potentially a wide variety of solid tumors including soft tissue sarcoma, head and neck cancers, liver cancers, prostate cancer, breast cancer, glioblastoma, etc., via multiple routes of administration.
NBTXR3 is being evaluated in: Soft tissue sarcoma (STS), head and neck cancers, prostate cancer, and liver cancers (primary and metastases). Additionally, head and neck cancer and rectal cancer trials led by Nanobiotix's Taiwanese partner, PharmaEngine, are underway in the Asia Pacific region.
The Company is also running research programs in immuno-oncology, with its lead product NBTXR3, which could have the potential to bring a new dimension to cancer immunotherapies. Nanobiotix received FDA's approval to launch a clinical study of NBTXR3 activated by Radiotherapy in combination with anti-PD1 antibody in lung, and head and neck cancer patients in the U.S.
Nanobiotix is listed on the regulated market of Euronext in Paris (ISIN: FR0011341205, Euronext ticker: NANO, Bloomberg: NANO: FP). The Company's Headquarters are based in Paris, France, with a U.S. affiliate in Cambridge, MA.
Contact
Nanobiotix
Sarah Gaubert
Director, Communications & Public Affairs
+33 (0)1 40 26 07 55
sarah.gaubert@nanobiotix.com / contact@nanobiotix.com
Noel Kurdi
Director, Investor Relations
+1 (646) 241-4400
noel.kurdi@nanobiotix.com / investors@nanobiotix.com
Media relations
France - Springbok Consultants
Marina Rosoff
+33 (0)6 71 58 00 34
marina@springbok.fr
United States - RooneyPartners
Marion Janic
+1 (212) 223-4017
mjanic@rooneyco.com
Disclaimer
This press release contains certain forward-looking statements concerning Nanobiotix and its business. Such forward-looking statements are based on assumptions that Nanobiotix considers to be reasonable. However, there can be no assurance that the estimates contained in such forward-looking statements will be verified, which estimates are subject to numerous risks including the risks set forth in the reference document of Nanobiotix filed with the French Financial Markets Authority (Autorite des Marches Financiers) under number D.17-0470 on April 28, 2017 (a copy of which is available on www.nanobiotix.com) and to the development of economic conditions, financial markets and the markets in which Nanobiotix operates. The forward-looking statements contained in this press release are also subject to risks not yet known to Nanobiotix or not currently considered material by Nanobiotix. The occurrence of all or part of such risks could cause actual results, financial conditions, performance or achievements of Nanobiotix to be materially different from such forward-looking statements.
This press release and the information that it contains do not constitute an offer to sell or subscribe for, or a solicitation of an offer to purchase or subscribe for, Nanobiotix shares in any country. At the moment NBTXR3 does not bear a CE mark and is not permitted to be placed on the market or put into service until NBTXR3 has obtained a CE mark.
Copyright 2018 ACN Newswire. All rights reserved. www.acnnewswire.com